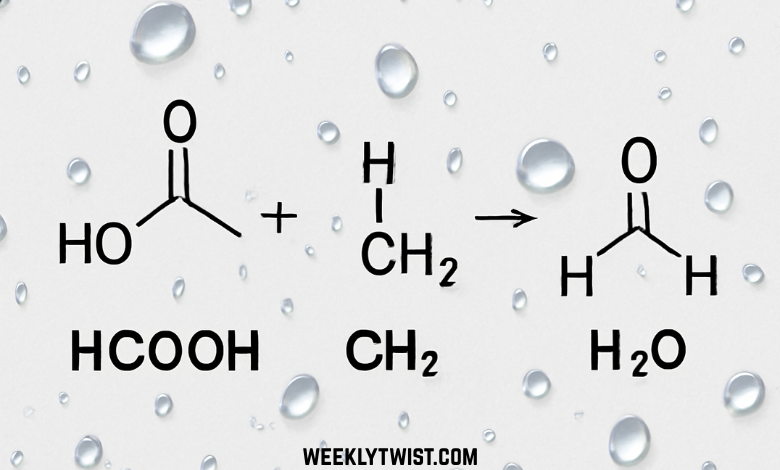
In the vast landscape of organic chemistry, the combination of molecules often dictates the chemical behavior and characteristics of a reaction. One such intriguing molecular trio is HCOOH CH2 H2O, a set involving formic acid (HCOOH), the methylene group (CH2), and water (H2O). Though simple in its individual components, this combination plays a vital role in various industrial processes, chemical reactions, and research methodologies. In this article, we will explore the significance, reactivity, and applications of this molecule triad and its components in both theoretical and practical contexts.
The Components: Breaking Down HCOOH CH2 H2O
To understand the reactivity and utility of HCOOH CH2 H2O, it is essential first to look at the individual components of the combination: formic acid (HCOOH), methylene group (CH2), and water (H2O).
1. Formic Acid (HCOOH)
Formic acid is one of the simplest carboxylic acids, containing a single carboxyl group (COOH). It is highly reactive and has a wide range of applications across multiple industries. As a weak acid, formic acid can donate a proton (H⁺) in aqueous solutions, making it a significant participant in acid-base chemistry. Formic acid’s reactivity allows it to engage in various reactions such as esterification, oxidation, and polymerization.
In the combination HCOOH CH2 H2O, formic acid acts as a reactant, often involved in oxidation reactions or as part of esterification reactions. Due to its simplicity and reactivity, formic acid plays a pivotal role in synthetic chemistry.
2. Methylene Group (CH2)
The methylene group (CH2) is a structural unit commonly found in a variety of organic molecules. Though not typically existing as a standalone molecule in reactions, the methylene group is a fundamental building block in organic chemistry. It is often derived from compounds like formaldehyde (CH2O) or methanol (CH3OH). In the context of HCOOH CH2 H2O, the methylene group often originates from formaldehyde, which acts as an intermediate in many industrial and laboratory processes.
The role of CH2 in HCOOH CH2 H2O combinations may involve intermediate synthesis or the formation of more complex molecules. Formaldehyde, a common derivative, is used in the production of resins, plastics, and textiles, where the methylene group serves as a crosslinking agent.
3. Water (H2O)
Water is a universal solvent, but it also plays a critical role beyond dissolution. In chemical reactions, water is involved in processes such as hydrolysis, hydration, and in influencing equilibrium reactions. In the context of HCOOH CH2 H2O, water is often present either as a reactant, byproduct, or solvent. Water’s role in the reactions involving formic acid and methylene compounds is multifaceted:
- In esterification reactions, water is typically produced as a byproduct.
- In oxidation reactions, water can be both a product and a medium for the reaction.
- Water’s polarity and high heat capacity allow it to stabilize reaction conditions and modulate reaction rates.
Reactions Involving HCOOH CH2 H2O
The combination of formic acid (HCOOH), methylene (CH2), and water (H2O) leads to several important organic reactions that are widely used in industrial and laboratory applications.
1. Esterification Reactions
Esterification is a classic organic reaction where an acid (in this case, formic acid) reacts with an alcohol (derived from methylene compounds like methanol) to form an ester. This process typically produces water as a byproduct. In industrial applications, esterification using formic acid is common in the production of formates—compounds used as solvents, preservatives, and in chemical synthesis.
For example, when formic acid (HCOOH) reacts with methanol (CH3OH), the result is the formation of methyl formate and water: HCOOH+CH3OH→HCOOCH3+H2O\text{HCOOH} + \text{CH}_3\text{OH} \rightarrow \text{HCOOCH}_3 + \text{H}_2\text{O}HCOOH+CH3OH→HCOOCH3+H2O
This reaction is a critical step in the production of esters used in fragrances, solvents, and plastics.
2. Formaldehyde Production
The combination of formic acid and methylene (CH2) plays an essential role in the production of formaldehyde (CH2O). Formaldehyde is a key industrial compound used in the manufacture of plastics, textiles, and resins. This reaction often occurs under heat or in the presence of a catalyst. Water is typically involved in moderating the reaction and controlling the rate of formaldehyde formation. HCOOH+CH2→CH2O+H2O\text{HCOOH} + \text{CH}_2 \rightarrow \text{CH}_2\text{O} + \text{H}_2\text{O}HCOOH+CH2→CH2O+H2O
In addition to industrial processes, formaldehyde is used as a disinfectant, preservative, and in the production of phenolic resins for the automotive and construction industries.
3. Oxidation Reactions
Formic acid can also undergo oxidation reactions in the presence of water and various catalysts. This process is important in various applications, including fuel cells and organic synthesis. For example, formic acid can be oxidized to produce carbon dioxide and water, releasing energy in the process. This reaction is part of the ongoing research into alternative energy sources, especially in the development of direct formic acid fuel cells. HCOOH+O2→CO2+H2O\text{HCOOH} + \text{O}_2 \rightarrow \text{CO}_2 + \text{H}_2\text{O}HCOOH+O2→CO2+H2O
Such oxidation reactions are central to renewable energy technologies and environmental sustainability.
Applications of HCOOH CH2 H2O in Industry
The combination of HCOOH CH2 H2O is not just limited to academic studies but plays a crucial role in a variety of industrial applications, particularly those involving formic acid as a starting material or intermediary.
1. Leather Tanning
In the leather industry, formic acid is commonly used in the tanning process. Formic acid’s antibacterial properties make it ideal for preserving leather and controlling microbial growth during processing. The reaction between formic acid and water, along with methylene derivatives, can help in modifying leather’s properties to make it more durable and resistant to decay.
2. Rubber Coagulation
Formic acid is also used in the rubber industry, particularly in the coagulation process. When combined with methylene compounds, formic acid helps to control the coagulation process, ensuring that the rubber maintains its desired consistency and elasticity.
3. Preservatives and Biocides
Formic acid and its derivatives, often in combination with methylene groups and water, are also used as preservatives in the food industry. Formates derived from formic acid are used to control microbial growth in food products, ensuring extended shelf life and food safety.
Conclusion
The combination of HCOOH CH2 H2O, involving formic acid, methylene, and water, is a foundational trio in organic chemistry, with wide-ranging applications across multiple industries. From esterification reactions to the production of formaldehyde and its industrial uses, this molecular combination drives numerous chemical processes that are vital to modern industry. The versatility of these reactions, coupled with the active role of water in mediating the reactions, underpins the importance of this trio in both laboratory research and commercial applications.
The study and application of HCOOH CH2 H2O not only advance the understanding of organic chemistry but also enhance the efficiency and sustainability of industrial processes, ranging from leather tanning to biocide production. As research continues, new applications and reactions involving this molecular combination will undoubtedly emerge, further cementing its role in the future of chemistry and industry.